A7 - Endocannabinoid signalling in the hypothalamic arcuate nucleus: Identification of novel players and mechanisms controlling energy homeostasis
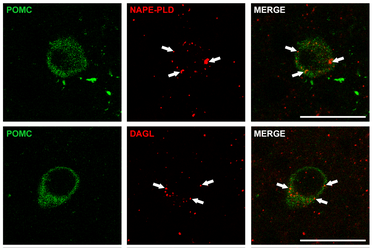
We want to understand how endocannabinoids stimulate overeating. The hypothalamic arcuate nucleus (ARC) harbours two prominent groups of neurons: the Agouti-related protein (AgRP) and neuropeptide Y (NPY) co-expressing neurons, which when activated rapidly promote food intake and decrease energy expenditure, while proopiomelanocortin (POMC) neurons drive gradual onset of satiety and increase energy expenditure. Indeed, maintenance of energy homeostasis is archived by reciprocal regulation of AgRP/NPY and POMC neurons in the ARC, as induced by humoral signals derived from the blood stream and through modulation of local synaptic input organisation. However, control of energy homeostasis is disrupted in the course of diet-induced obesity and by exogenous substances, such as cannabinoids. For example, activation of the cannabinoid receptor type 1 (CB1) by exogenous cannabinoids provokes a robust feeding response, despite the respective individual resides in a state of satiety. Endocannabinoids are endogenous lipids that generally elicit the same biological effects via CB1 when compared to exogenous cannabinoids. Moreover, endocannabinoids are able to transmit physiological effects independently from CB1 activation. Thus, our research will focus on novel mechanistic insights on how endocannabinoids in the ARC might promote metabolic effects. In this context, preliminary work to our project demonstrated that endocannabinoids are present in the ARC and thus might affect POMC and AgRP/NPY neurons (Figure 1). Our overall goal is to dissect the respective mechanisms by which local endocannabinoids will contribute to orchestration of whole-body energy homeostasis.
Figure 1. Enzymes involved in biosynthesis of endocannabinoids are present in the mouse ARC. Immunofluorescence in transgenic POMC-GFP reporter mice revealed that POMC neurons (green) express distinct endocannabinoid producing enzymes, such as NAPE-PLD (upper panel: red, arrows) and DAGL (lower panel: red, arrows). Bars = 25 mm.
García-Cáceres C, Balland E, Prevot V, Luquet S, Woods SC, Koch M, Horvath TL, Yi CX, Chowen JA, Verkhratsky A, Araque A, Bechmann I, Tschöp MH. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat Neurosci. 2019 Jan;22(1):7-14.
Horn H, Böhme B, Dietrich L, Koch M. Endocannabinoids in Body Weight Control. Pharmaceuticals (Basel). 2018 May 30;11(2).
Quarta C, Clemmensen C, Zhu Z, Yang B, Joseph SS, Lutter D, Yi CX, Graf E, García-Cáceres C, Legutko B, Fischer K, Brommage R, Zizzari P, Franklin BS, Krueger M, Koch M, Vettorazzi S, Li P, Hofmann SM, Bakhti M, Bastidas-Ponce A, Lickert H, Strom TM, Gailus-Durner V, Bechmann I, Perez-Tilve D, Tuckermann J, Hrabě de Angelis M, Sandoval D, Cota D, Latz E, Seeley RJ, Müller TD, DiMarchi RD, Finan B, Tschöp MH. Molecular Integration of Incretin and Glucocorticoid Action Reverses Immunometabolic Dysfunction and Obesity. Cell Metab. 2017;26:620-32.
Koch M. Cannabinoid receptor signaling in central regulation of feeding behavior: a mini-review. Front Neurosci. 2017;11:293.
Morozov YM, Koch M, Rakic P, Horvath TL. Cannabinoid type 1 receptor-containing axons innervate hunger-promoting AgRP/NPY neurons in the mouse arcuate nucleus. Mol Metabol. 2017;6:374-81.
Jin S, Kim JK, Kim KK, Park JW, Koch M, Horvath TL, Lee BJ. Hypothalamic TLR2 triggers sickness behavior via a microglia-neuronal axis. Sci Rep. 2016;6:29424.
Horvath TL, Kim JG, Sun BH, Dietrich MO, Koch M, Yao GQ, Diano S, Insogna K. AgRP neurons regulate bone homeostasis. Cell Rep. 2015;13:8-14.
Koch M, Ferreirós N, Geisslinger G, Dehghani, Korf HW. Rhythmic control of endocannabinoids in the rat pineal gland. Chronobiol Int. 2015;10:1-6.
Koch M, Varela L, Kim JG, Kim JD, Hernández-Nuño F, Simonds SE, Castorena CM, Vianna CR, Elmquist JK, Morozov YM, Rakic P, Bechmann I, Cowley MA, Szigeti-Buck K, Gao X-B, Dietrich MO, Diano S, Horvath TL. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519:45-50.
Kim JG, Suyama S, Koch M, Szigeti K, Gao Y, Garcia-Caceres C, Yi CX, Chowen J, Tschop MH, Horvath TL. Leptin receptors in astrocytes control synaptic input organization of melanocortin cells and metabolic adaptations. Nat Neurosci. 2014;17:908-10.
Koch M, Horvath TL. Neuronal circuitries that regulate food intake and energy metabolism. Mol Psychiatry. 2014;9:752-61.
Kallendrusch S, Kremzow S, Nowicki M, Hobusch C, Merkwitz C, Winkelmann R, Benz AH, Kraft R, Bechmann I, Dehghani F, Koch M. The GPR55 ligand l-α-lysophosphatidylinositol exerts microglia-dependent neuroprotection after excitotoxic lesion. Glia. 2013;61:1822–31.
Morozov YM, Dominguez MH, Varela L, Shanabrough M, Koch M, Horvath TL, Rakic P. Antibodies to cannabinoid type 1 receptor co-react with stomatin-like protein 2 in mouse brain mitochondria. Eur J Neurosci. 2013;38:2341–8.
Kallendrusch S, Hobusch C, Ehrlich A, Nowicki M, Ziebell S, Geisslinger G, Koch M, Dehghani F. Intrinsic up-regulation of 2-AG favors an area specific neuronal survival in different in vitro models of neuronal damage. PLoS One. 2012;7:e51208.
Koch M, Horvath TL. Reward aspects of gastrointestinal hormones mediated by brain g protein-coupled receptors. Biol Psych 2012;72:340-2.
Kallendrusch S, Hobusch C, Ehrlich A, Ziebell S, Ueda N, Geisslinger G, Koch M, Dehghani F. Site-specific and time-dependent activation of the endocannabinoid system after transection of long-range projections. PLoS One. 2012;7:e33537.
Grabiec U, Koch M, Kallendrusch S, Kraft R, Hill K, Merkwitz C, Ghadban C, Lutz B, Straiker A, Dehghani F. The endocannabinoid N-arachidonoyldopamine (NADA) exerts neuroprotective effects after excitotoxic neuronal damage via cannabinoid receptor 1 (CB(1)). Neuropharmacol. 2012;62:1797-807.
Koch M, Kreutz S, Böttger C, Grabiec U, Ghadban C, Korf HW, Deghani F. The cannabinoid WIN 55,212-2-mediated protection of dentate gyrus granule cells is driven by CB1 receptors and modulated by TRPA1 and Cav2.2 channels. Hippocampus. 2011;21:554-64.
Koch M, Kreutz S, Böttger C, Ghadban C, Korf HW, Dehghani F. Palmitoylethanolamide (PEA) protects dentate gyrus granule cells via peroxisome proliferator-activated receptor (PPAR)-alpha. Neurotoxicity Res. 2011;19:330-40.