B2 -The miRNA and single cell perspectives of the E2F1/inflammation/autophagy network in human adipose tissue
Autophagy plays an important role in the development of adipose tissue dysfunction. In human obesity, activation of autophagy is regulated by E2F1 and specific miRNAs. The project now takes the E2F1-autophagy/inflammation network to the single-cell level. Single-nucleus RNA-sequencing (sNucSeq) will be used to capture the cellular repertoire and the single cell-type impact of the E2F1 network. The project further aims to understand how E2F1 signifies changes in the repertoire of adipose tissue miRNAs and to what degree these are reflected in circulating miRNAs. Finally, it will be determined if these E2F1 network components constitute biomarkers to predict clinical outcomes in response to bariatric surgery, such as weight loss, metabolic improvement, diabetes remission, and a decrease in cardiovascular risk parameters.
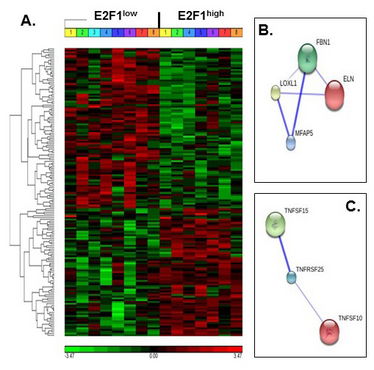
Figure 1. Increased human AT E2F1 expression and E2F1 mRNA stability. A. Hierarchical clustering of differentially expressed genes. Differential transcriptome between E2F1high and E2F1low is presented, divided to clusters of upregulated and downregulated genes. B and C. Selected pathway analyses, using STRING10© protein-interaction database. Shown are predicted and/or experimentally established pathways of ECM-related genes and TNF-related superfamily genes, differentially expressed in our cohort. Line thickness represents confidence level of the interaction.
Landgraf K, Klöting N, Gericke M, Maixner N, Guiu-Jurado E, Scholz M, Witte AV, Beyer F, Schwartze JT, Lacher M, Villringer A, Kovacs P, Rudich A, Blüher M, Kiess W, Körner A.The Obesity-Susceptibility Gene TMEM18 Promotes Adipogenesis through Activation of PPARG.Cell Rep. 2020 Oct 20;33(3):108295.
Mattar P, Sanhueza S, Yuri G, Briones L, Perez-Leighton C, Rudich A, Lavandero S, Cifuentes M. Calcium-Sensing Receptor in Adipose Tissue: Possible Association with Obesity-Related Elevated Autophagy. Int J Mol Sci. 2020 Oct 15;21(20):7617.
Zacharia A, Saidemberg D, Mannully CT, Kogan NM, Shehadeh A, Sinai R, Zucker A, Bruck-Haimson R, Goldstein N, Haim Y, Dani C, Rudich A, Moussaieff A. Distinct infrastructure of lipid networks in visceral and subcutaneous adipose tissues in overweight humans. Am J Clin Nutr. 2020 Aug 7;nqaa195.
Maixner N, Pecht T, Haim Y, Chalifa-Caspi V, Goldstein N, Tarnovscki T, Liberty IF, Kirshtein B, Golan R, Berner O, Monsonego A, Bashan N, Blüher M, Rudich A. A TRAIL-TL1A Paracrine Network Involving Adipocytes, Macrophages and lymphocytes Induces Adipose Tissue Dysfunction Downstream of E2F1 in Human Obesity. Diabetes. 2020 Jul 30;db191231.
Goldstein N, Kezerle Y, Gepner Y, Haim Y, Pecht T, Gazit R, Polischuk V, Liberty IF, Kirshtein B, Shaco-Levy R, Blüher M, Rudich A. Higher Mast Cell Accumulation in Human Adipose Tissues Defines Clinically Favorable Obesity Sub-Phenotypes. Cells . 2020 Jun 20;9(6):E1508.
Hadadi-Bechor S, Haim Y, Pecht T, Gat R, Tarnovsky T, Gericke M, Rudich A, Autophagy differentially regulates macrophage lipid handling depending on the lipid substrate (oleic acid vs. acetylated-LDL) and inflammatory activation state., Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Sep 11:158527
Heinitz S, Gebhardt C, Piaggi P, Krüger J, Heyne H, Weiner J, Heiker JT, Stumvoll M, Blüher M, Baier L, Rudich A, Kovacs P, Tönjes A. Atg7-knockdown reduces chemerin secretion in murine adipocytes. J Clin Endocrinol Metab. 2019 Jun 21. pii: jc.2018-01980.
Gepner Y, Shelef I, Komy O, Cohen N, Schwarzfuchs D, Bril N, Rein M, Serfaty D, Kenigsbuch S, Zelicha H, Yaskolka Meir A, Tene L, Bilitzky A, Tsaban G, Chassidim Y, Sarusy B, Ceglarek U, Thiery J, Stumvoll M, Blüher M, Stampfer MJ, Rudich A, Shai I. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat Content. EBioMedicine. 2019 May 29. pii: S2352-3964(19)30358-5.
Goldstein N, Haim Y, Mattar P, Hadadi-Bechor S, Maixner N, Kovacs P, Blüher M, Rudich A. Leptin stimulates autophagy/lysosome-related degradation of long-lived proteins in adipocytes. Adipocyte. 2019 Jan 24.
Boura-Halfon S, Pecht T, Jung S, Rudich A. Obesity and dysregulated central and peripheral Macrophage-neuron crosstalk. Eur J Immunol. 2018 Nov 8.
Haim Y, Blüher M, Konrad D, Goldstein N, Klöting N, Harman-Boehm I, Kirshtein B, Ginsberg D, Tarnovscki T, Gepner Y, Shai I, Rudich A. ASK1 (MAP3K5) is transcriptionally upregulated by E2F1 in adipose tisue in obesity, molecularly defining a human dys-metabolic obese phenotype. Mol Metab. 2017;6:725-36.
Bechor S, Nachmias D, Elia N, Haim Y, Vatarescu M, Leikin-Frenkel A, Gericke M, Tarnovsky T, Las G, Rudich A. Adipose tissue conditioned media support macrophage lipid-droplet biogenesis by interfering with autophagic flux. Biochim Biophys Acta. 2017;1862:1001-12.
Pecht T, Haim Y, Bashan N, Shapiro H, Harman-Boehm I, Kirshtein B, Clement K, Shai I, Rudich A. Circulating blood monocyte subclasses and lipid-laden adipose tissue macrophages in human obesity. PLoS One. 2016;11:e0159350.
Slutsky N, Vatarescu M, Haim Y, Goldstein N, Kirshtein B, Harman-Boehm I, Gepner Y, Shai I, Bashan N, Blüher M, Rudich A. Decreased adiponectin links elevated adipose tissue autophagy with adipocyte endocrine dysfunction in obesity. Int J Obes (Lond). 2016;40:912-20.
Haim Y, Blüher M, Slutsky N, Goldstein N, Klöting N, Harman-Boehm I, Kirshstein B, Ginsberg D, Gericke M, Guiu Jurado E, Kovsan J, Tarnovscki T, Kachko L, Bashan N, Gepner Y, Shai I, Rudich A. Elevated autophagy gene expression in adipose tissue of humans: A potential non-cell-cycle-dependent function of E2F1. Autophagy. 2015;11:2074-88.
Stienstra R, Haim Y, Riahi Y, Netea M, Rudich A, Leibowitz G. Autophagy in adipose tissue and the beta cell: implications for obesity and diabetes. Diabetologia. 2014;57:1505-16.
Haim Y, Tarnovscki T, Bashari D, Rudich A. A chromatin immunoprecipitation (ChIP) protocol for use in whole human adipose tissue. Am J Physiol Endocrinol Metab. 2013;305:E1172-7.
Pecht T, Gutman-Tirosh A, Bashan N, Rudich A. Periperal blood leucocyte subclasses as potential biomarkers of adipose tissue inflammation and obesity subphenotypes in humans. Obes Rev. 2014;15:322-37.
Rudich A, Klip A. Putting Rac1 on the path to glucose uptake. Diabetes. 2013;62:1831-2.
Tirosh A, Golan R, Harman-Boehm I, Henkin Y, Schwarzfuchs D, Rudich A, Kovsan J, Fiedler G, Blüher M, Stumvoll M, Thiery J, Stampfer M, Shai I. Renal function following three distinct weight loss dietary strategies during 2 years of randomized controlled trial. Diabetes Care. 2013;36:2225-32.
Wolak T, Sion-Vardi N, Novack V, Greenberg G, Szendro G, Tarnovscki T, Nov O, Shelef I, Paran E, Rudich A. N-terminal osteopontin, rather than full-length protein or C-terminal fragment, associates with carotid plaque inflammation in hypertensive patients. Am J Hypertension. 2013;26:326-33.
Nov O, Shapiro H, Ovadia H, Tarnovscki T, Dvir I, Shemesh E, Kovsan J, Shelef I, Carmi Y, Voronov E, Apte RN, Lewis E, Haim Y, Konrad D, Bashan N, Rudich A. Role of IL-1β in adipose tissue inflammation, expandability, and fat–liver crosstalk in obesity. PLoS One. 2013;8:e53626.
Shapiro H, Pecht T, Shaco-Levy R, Harman-Boehm I, Kirshtein B, Kuperman Y, Chen A, Blüher M, Shai I, Rudich A. Adipose tissue foam cells are present in human obesity. J Clin Endocrinol Metab. 2013;98:1173-81.
Golan R, Shelef I, Rudich A, Gepner Y, Shemesh E, Chassidim Y, Harman-Boehm I, Henkin Y, Schwarzfuchs D, Ben Avraham S, Witkow S, Tangi-Rosental O, Liberty IF, Sarusi B, Stampfer MJ, Shai I. Abdominal superficial subcutaneous fat – a putative distinct protective fat sub-depot in type 2 diabetes. Diabetes Care. 2012;35:640-7.
Blüher M, Rudich A, Klöting N, Golan R,Henkin Y, Rubin E, Schwarzfuchs D, Gepner Y, Stampfer M, Fiedler M, Thiery J, Stumvoll M, Shai I. Two patterns of adipokine and other biomarker dynamics in a long term weight loss intervention. Diabetes Care. 2012;35:342-9.
Klionsky DJ, Abdalla FC, Abeliovich H, [...], Rudich A, [...], Zuckerbraun B. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445-544.
Maixner N, Kovsan J, Harman-Boehm I, Blüher M, Bashan N, Rudich A. Adipose tissue autophagy. Obesity Facts. 2012;5:710-21.
Kovsan J, Bluher M, Tarnovscki T, Kloting N, Kirshtein B, Madar L, Shai I, Golan R, Harman-Boehm I, Schon MR, Greenberg AS, Elazar Z, Bashan N, Rudich A. Altered autophagy in human adipose tissues in obesity. J Clin Endocrinol Metab. 2011;96:E268-77.
Ovadia H, Haim Y, Nov O, Almog O, Kovsan J, Bashan N, Benhar M, Rudich A. Increased adipocytes S-nitrosylaion targets the anti-lipolytic action of insulin: relevance to adipose tissue dysfunction in obesity. J Biol Chem. 2011;286:30433-43.
Kovsan J, Bashan N, Greenberg AS, Rudich A. Potential role of autophagy in modulation of lipid metabolism. Am J Physiol (Endocrinol Metab). 2010;298:E1-E7.
Nov O, Kohl A, Lewis EC, Bashan N, Dvir I, Ben-Shlomo S, Fishman S, Wueest S, Konrad D, Rudich A. Interleukin-1β may mediate insulin resistance in liver-derived cells in response to adipocyte inflammation. Endocrinology. 2010;151:4247-56.
Bluher M, Bashan N, Shai I, Harman-Boehm I, Tarnovscki T, Avinaoch E, Stumvoll M, Dietrich A, Kloting N, Rudich A. Activated Ask1-MKK4-p38MAPK/JNK stress signaling pathway in human omental fat tissue may link macrophage infiltration to whole-body Insulin sensitivity. J Clin Endocrinol Metab. 2009;94:2507-15.
Harman-Boehm I, Bluher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, Shai I, Kloting N, Stumvoll M, Bashan N, Rudich A. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity.J Clin Endocrinol Metab. 2007;92:2240-7.
Bashan N, Dorfman K, Tarnovscki T, Harman-Boehm I, Liberty IF, Bluher M, Ovadia S, Maymon-Zilberstein T, Potashnik R, Stumvoll M, Avinoac h E,Rudich A. Mitogen-activated protein kinases, inhibitory-kappaB kinase, and insulin signaling in human omental versus subcutaneous adipose tissue in obesity. Endocrinology. 2007;148:2955-62.
PostDoc
Yulia Haim
|
Office address:
|
|
|
Phone (lab or office):
E-mail:
|
|
|
Project description:
|
She leads the optimization of the sNucSeq technique for human adipose tissue, performs these experiments and bulk RNA-sequencing.
|
Doctoral Researcher
Nataly Makarenkov
|
Office address:
|
|
|
Phone (lab or office):
E-mail:
|
|
|
Research subject:
|
Mediatory role of adipose tissue-derived miRNAs in sub-phenotypes of obesity and obesity-related NAFLD. Her thesis focuses on miRNA analysis including the "wet-lab" experiments and miRNA bioinformatics.
|
Doctoral Researcher
Alon Zemer
|
Adresse (Büro):
|
|
|
Telefon:
E-mail:
|
|
|
Thema des Promotionsprojektes:
|
|