C4 - Structural studies on adipokines and other proteins related to obesity
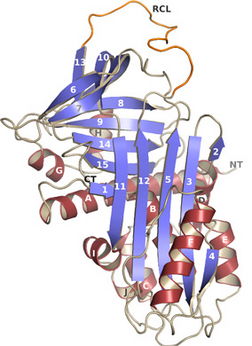
We investigate the crystal structures and the molecular mode of action of several proteins related to obesity. In previous work we analyzed the structural basis of the mechanism of inhibition of the serine protease inhibitor vaspin on its target protease kallikrein 7 (Klk7) by co-crystal structures. Further work in this subproject focuses on the interaction with heparin, which enhances the inhibitory potential of vaspin towards Klk7. Furthermore, we aim to study the vaspin–Klk7 interaction by the determination of crystal structures of the vaspin-Klk7 complex and of complexes with peptides mimicking the protease reactive center loop. Furthermore, mutational analysis on putative exosites is used to characterize the interaction.
Figure 1. Crystal structure of the serpin vaspin.
Frenster JD, Stephan G, Ravn-Boess N, Bready D, Wilcox J, Kieslich B, Wilde C, Sträter N, Wiggin GR, Liebscher I, Schöneberg T, Placantonakis DG. Functional impact of intramolecular cleavage and dissociation of adhesion G protein-coupled receptor GPR133 (ADGRD1) on canonical signaling. J Biol Chem. 2021 May 19:100798.
Hanke S, Tindall CA, Pippel J, Ulbricht D, Pirotte B, Reboud-Ravaux M, Heiker JT, Sträter N. Structural Studies on the Inhibitory Binding Mode of Aromatic Coumarinic Esters to Human Kallikrein-Related Peptidase 7. J Med Chem. 2020 Jun 11;63(11):5723-5733.
Tindall CA, Dommel S, Riedl V, Ulbricht D, Hanke S, Sträter N, Heiker JT. Membrane Phospholipids and Polyphosphates as Cofactors and Binding Molecules of SERPINA12 (vaspin). Molecules. 2020 Apr 24;25(8):1992.
Bhattarai S, Pippel J, Scaletti E, Idris R, Freundlieb M, Rolshoven G, Renn C, Lee SY, Abdelrahman A, Zimmermann H, El-Tayeb A, Müller CE, Sträter N. 2-Substituted α,β-Methylene-ADP Derivatives: Potent Competitive Ecto-5'-nucleotidase (CD73) Inhibitors with Variable Binding Modes. J Med Chem. 2020 Feb 28.
Ulbricht D, Tindall CA, Oertwig K, Hanke S, Sträter N, Heiker JT. Kallikrein-related peptidase 14 is the second KLK protease targeted by the serpin vaspin. Biological Chemistry 2018 Feb 1.
Oertwig K, Ulbricht D, Hanke S, Pippel J, Bellmann-Sickert K, Sträter N, Heiker JT. Glycosylation of human vaspin (SERPINA12) and its impact on serpin activity, heparin binding and thermal stability. Biochim Biophys Acta. 2017;1865:1100-3.
Ulbricht D, Oertwig K, Arnsburg K, Saalbach A, Pippel J, Sträter N, Heiker JT. Basic Residues of β-Sheet A Contribute to Heparin Binding and Activation of Vaspin (Serpin A12). J Biol Chem. 2017;292:994-1004.
Pippel J, Küttner EB, Ulricht D, Daberger J, Schultz S, Heiker JT, Sträter N. Crystal structure of cleaved vaspin (serpinA12). Biol Chem. 2016;397:111-23.
Ulbricht D, Pippel J, Schultz S, Meier R, Sträter N, Heiker JT. A unique serpin P1' glutamate and a conserved beta-sheet C arginine are key residues for activity, protease recognition and stability of serpinA12 (vaspin). Biochem J. 2015;470:357-67.
Heiker JT, Klöting N, Kovacs P, Kuettner EB, Sträter N, Schultz S, Kern M, Stumvoll M, Blüher M, Beck-Sickinger AG. Vaspin inhibits kallikrein 7 by serpin mechanism. Cell Mol Life Sci. 2013;70:2569-83.