Z3 - Proteome and lipidome analysis by mass spectrometry and NMR
The Z3 project will provide a proteomics and metabolomics (particularly lipidomics) platform comprising a very broad collection of state of the art approaches in mass spectrometry and NMR. For the characterization of samples from obese patients, function analyses of the global proteome (targeted detection of adipokines in serum, global data independent serum proteomics), the lipid composition as well as the isotopic flux in the metabolome will be performed. Additionally, spatially-resolved data of metabolite distributions within the tissues of interest will be provided by MALDI MS imaging.
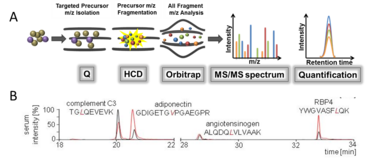
Figure 1: SRM method which enables the selective determination of adipozytokins. (A) This method uses two different mass filters and quantitative data are obtained by characteristic "mass transitions". (B) Oberbach et al. (2015) provided evidence that this method can be used to quantify at least 4 different serum proteins.
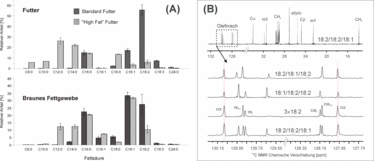
Figure 2: Impact of the supplied diet on the fatty acyl composition of brown adipose tissue. (A) The characteristic fatty acid compositions of the diet and the adipose tissue show pronounced differences. (B) By using 13C NMR the positions of the individual fatty acyl residues can be determined. In (2A) the impact of different diets on the fatty acyl composition of brown adipose tissue (from mice) is shown. In the used x:y nomenclature "x" denotes the number of carbon atoms in the fatty acid while "y" denotes the number of double bonds. The determination of the positions of the different acyl residues can be achieved by 13C NMR spectroscopy (2B). In the majority of cases sn-1,3- and sn-2 positions can be differentiated.
Krieg L, Didt K, Karkossa I, Bernhart SH, Kehr S, Subramanian N, Lindhorst A, Schaudinn A, Tabei S, Keller M, Stumvoll M, Dietrich A, von Bergen M, Stadler PF, Laurencikiene J, Krüger M, Blüher M, Gericke M, Schubert K, Kovacs P, Chakaroun R, Massier L. Gut. 2021 Oct 1:gutjnl-2021-324603.
Yaskolka Meir A, Tuohy K, von Bergen M, Krajmalnik-Brown R, Heinig U, Zelicha H, Tsaban G, Rinott E, Kaplan A, Aharoni A, Zeibich L, Chang D, Dirks B, Diotallevi C, Arapitsas P, Vrhovsek U, Ceglarek U, Haange SB, Rolle-Kampczyk U, Engelmann B, Lapidot M, Colt M, Sun Q, Shai I. The Metabolomic-Gut-Clinical Axis of Mankai Plant-Derived Dietary Polyphenols. Nutrients. 2021 May 30;13(6):1866.
Chen J, Haase N, Haange SB, Sucher R, Münzker J, Jäger E, Schischke K, Seyfried F, von Bergen M, Hankir MK, Krügel U, Fenske WK. Roux-en-Y gastric bypass contributes to weight loss-independent improvement in hypothalamic inflammation and leptin sensitivity through gut-microglia-neuron-crosstalk. Mol Metab. 2021 Mar 16:101214.
Streidl T, Karkossa I, Segura Muñoz RR, Eberl C, Zaufel A, Plagge J, Schmaltz R, Schubert K, Basic M, Schneider KM, Afify M, Trautwein C, Tolba R, Stecher B, Doden HL, Ridlon JM, Ecker J, Moustafa T, von Bergen M, Ramer-Tait AE, Clavel T. The gut bacterium Extibacter muris produces secondary bile acids and influences liver physiology in gnotobiotic mice. Gut Microbes. 2021 Jan-Dec;13(1):1-21.
Rolle-Kampczyk U, Gebauer S, Haange SB, Schubert K, Kern M, Moulla Y, Dietrich A, Schön MR, Klöting N, von Bergen M, Blüher M. Accumulation of distinct persistent organic pollutants is associated with adipose tissue inflammation. Sci Total Environ. 2020 Dec 15;748:142458.
Le Duc D, Lin CC, Popkova Y, Yang Z, Akhil V, Çakir MV, Grunewald S, Simon JC, Dietz A, Dannenberger D, Garten A, Lemke JR, Schiller J, Blüher M, Nono Nankam PA, Rolle-Kampczyk U, von Bergen M, Kelso J, Schöneberg T. Reduced lipolysis in lipoma phenocopies lipid accumulation in obesity. Int J Obes (Lond). 2020 Nov 24.
Asimakopoulou A, Engel KM, Gassler N, Bracht T, Sitek B, Buhl EM, Kalampoka S, Pinoé-Schmidt M, van Helden J, Schiller J, Weiskirchen R. Deletion of Perilipin 5 Protects Against Hepatic Injury in Nonalcoholic Fatty Liver Disease via Missing Inflammasome Activation. Cells .2020 May 28;9(6):E1346.
Kalkhof S, Krieg L, Büttner P, Wabitsch M, Küntzel C, Friebe D, Landgraf K, Hanschkow M, Schubert K, Kiess W, Krohn K, Blüher M, von Bergen M, Körner A. In Depth Quantitative Proteomic and Transcriptomic Characterization of Human Adipocyte Differentiation Using the SGBS Cell Line. Proteomics.2020 May 8;e1900405.
Krieg L, Schaffert A, Kern M, Landgraf K, Wabitsch M, Beck-Sickinger AG, Koerner A, Blüher M, von Bergen M, Schubert K. An MRM-Based Multiplexed Quantification Assay for Human Adipokines and Apolipoproteins. Molecules. 2020 Feb 11;25(4). pii: E775.
Leppert B, Strunz S, Seiwert B, Schlittenbauer L, Schlichting R, Pfeiffer C, Röder S, Bauer M, Borte M, Stangl GI, Schöneberg T, Schulz A, Karkossa I, Rolle-Kampczyk UE, Thürmann L, von Bergen M, Escher BI, Junge KM, Reemtsma T, Lehmann I, Polte T. Maternal paraben exposure triggers childhood overweight development. Nat Commun. 2020 Feb 11;11(1):561.
Haange SB, Jehmlich N, Krügel U, Hintschich C, Wehrmann D, Hankir M, Seyfried F, Froment J, Hübschmann T, Müller S, Wissenbach DK, Kang K, Buettner C, Panagiotou G, Noll M, Rolle-Kampczyk U, Fenske W, von Bergen M. Gastric bypass surgery in a rat model alters the community structure and functional composition of the intestinal microbiota independently of weight loss. Microbiome. 2020 Feb 7;8(1):13.
Farag MA, Abdelwareth A, Sallam IE, El Shorbagi M, Jehmlich N, Fritz-Wallace K, Serena Schäpe S, Rolle-Kampczyk U, Ehrlich A, Wessjohann LA, von Bergen M. Metabolomics reveals impact of seven functional foods on metabolic pathways in a gut microbiota model. J Adv Res. 2020 Jan 3;23:47-59.
Miletić Vukajlović J, Drakulić D, Pejić S, Ilić TV, Stefanović A, Petković M, Schiller J., INCREASED PLASMA PHOSPHATIDYLCHOLINE / LYSOPHOSPHATIDYLCHOLINE RATIOS IN PATIENTS WITH PARKINSON'S DISEASE. Rapid Commun Mass Spectrom. 2019 Sep 13.
Zelicha H, Kaplan A, Yaskolka Meir A, Tsaban G, Rinott E, Shelef I, Tirosh A, Brikner D, Pupkin E, Qi L, Thiery J, Stumvoll M, Kloting N, von Bergen M, Ceglarek U, Blüher M, Stampfer MJ, Shai I. The Effect of Wolffia globosa Mankai, a Green Aquatic Plant, on Postprandial Glycemic Response: A Randomized Crossover Controlled Trial. Diabetes Care. 2019 Jul;42(7):1162-1169
Mandić AD, Woting A, Jaenicke T, Sander A, Sabrowski W, Rolle-Kampcyk U, von Bergen M, Blaut M. Clostridium ramosum regulates enterochromaffin cell development and serotonin release. Sci Rep. 2019 Feb 4;9(1):1177.
Ditz T, Schnapka-Hille L, Noack N, Dorow J, Ceglarek U, Niederwieser D, Schiller J, Fuchs B, Cross M. Phospholipase A2 products predict the hematopoietic support capacity of horse serum. Differentiation. 2018 Dec 6;105:27-32.
Leopold J, Popkova Y, Engel KM, Schiller J. Recent Developments of Useful MALDI Matrices for the Mass Spectrometric Characterization of Lipids. Biomolecules. 2018 Dec 13;8(4).
Vorselen D, van Dommelen SM, Sorkin R, Piontek MC, Schiller J, Döpp ST, Kooijmans SAA, van Oirschot BA, Versluijs BA, Bierings MB, van Wijk R, Schiffelers RM, Wuite GJL, Roos WH.The fluid membrane determines mechanics of erythrocyte extracellular vesicles and is softened in hereditary spherocytosis. Nat Commun. 2018 Nov 23;9(1):4960.
Leopold J, Popkova Y, Engel KM, Schiller J. Visualizing phosphatidylcholine via mass spectrometry imaging: relevance to human health. Expert Rev Proteomics. 2018 Sep 21.
Kratochvil I, Hofmann T, Rother S, Schlichting R, Moretti R, Scharnweber D, Hintze V, Escher BI, Meiler J, Kalkhof S, von Bergen M. MEHP and MEOHP but not DEHP bind productively to the peroxisome proliferator-activated receptor γ. Rapid Commun Mass Spectrom. 2018 Aug 7.
Engel K, Griesinger H, Schulz M, Schiller J.Normal Phase versus Reversed Phase TLC to monitor Oxidized Phosphatidylcholines by TLC/Mass Spectrometry. Rapid Commun Mass Spectrom. 2018 Jul 18.
Schröter J, Fülöp A, Hopf C, Schiller J. The combination of 2,5-dihydroxybenzoic acid and 2,5-dihydroxyacetophenone matrices for unequivocal assignment of phosphatidylethanolamine species in complex mixtures. Anal Bioanal Chem. 2018 Mar;410(9):2437-2447.
Herbert D, Franz S, Popkova Y, Anderegg U, Schiller J, Schwede K, Lorz A, Simon JC, Saalbach A. High fat diet exacerbates early psoriatic skin inflammation independent of obesity: Saturated fatty acids as key players. J Invest Dermatol. 2018 Mar 29. pii: S0022-202X(18)31858-X.
Junge KM, Leppert B, Jahreis S, Wissenbach DK, Feltens R, Grützmann K, Thürmann L, Bauer T, Ishaque N, Schick M, Bewerunge-Hudler M, Röder S, Bauer M, Schulz A, Borte M, Landgraf K, Körner A, Kiess W, von Bergen M, Stangl GI, Trump S, Eils R, Polte T, Lehmann I. MEST mediates the impact of prenatal bisphenol A exposure on long-term body weight development. Clin Epigenetics. 2018 Apr 20;10:58.
Engel KM, Schiller J, Müller K, Dannenberger D, Jakop U. The phospholipid composition of kangaroo spermatozoa verified by mass spectrometric lipid analysis. Lipids. 2017;52:857-69.
Engel KM, Schiller J. A comparison of PC oxidation products as detected by MALDI-TOF and ESI-IT mass spectrometry. Chem Phys Lipids. 2017;203:33-45.
Popkova Y, Schiller J. TAG suppression by CsCl addition. Rapid Commun Mass Spectrom. 2017;31:411-8.
Schröter J, Süß R, Schiller J. MALDI-TOF MS to monitor the kinetics of phospholipase A2-digestion of oxidized phospholipids. Methods. 2016;104:41-7.
Stelzner K, Herbert D, Popkova Y, Lorz A, Schiller J, Gericke M, Klöting N, Blüher M, Franz S, Simon JC, Saalbach A. Free fatty acids sensitize dendritic cells to amplify TH1/TH17-immune responses. Eur J Immunol. 2016;46:2043-53.
Kühn T, Floegel A, Sookthai D, Johnson T, Rolle-Kampczyk U, Otto W, von Bergen M, Boeing H, Kaaks R. Higher plasma levels of lysophosphatidylcholine 18:0 are related to a lower risk of common cancers in a prospective metabolomics study. BMC Med. 2016;14:13.
Popkova Y, Meusel A, Breitfeld J, Schleinitz D, Hirrlinger J, Dannenberger D, Kovacs P, Schiller J. Nutrition-dependent changes of adipose tissue compositions monitored by NMR, MS and chromatographic methods. Anal Bioanal Chem. 2015;407:5123-33.
Klöting N, Hesselbarth N, Gericke M, Kunath A, Biemann R, Chakaroun R, Kosacka J, Kovacs P, Kern M, Stumvoll M, Fischer B, Rolle-Kampczyk U, Feltens R, Otto W, Wissenbach DK, von Bergen M, Blüher M. Di-(2-ethylhexyl)-phthalate (DEHP) causes impaired adipocyte function and alters serum metabolites. PLoS One. 2015;10:e0143190.
Herberth G, Offenberg K, Rolle-Kampczyk U, Bauer M, Otto W, Röder S, Grützmann K, Sack U, Simon JC, Borte M, von Bergen M, Lehmann I; LINA Study Group. Endogenous metabolites and inflammasome activity in early childhood and links to respiratory diseases. J Allergy Clin Immunol. 2015;136:495-7.
Fuchs B, Popkova Y, Süß R, Schiller J. Separation of (Phospho)lipids by thin-layer chromatography. In: Poole C. (Ed.): "Handbooks in Separation Science: Instrumental Thin-Layer Chromatography". Elsevier, Amsterdam, 2015, pp. 375-405. ISBN 978-0-12-417223-4 .
Schiller J, Fuchs B, Süß R, Popkova Y, Griesinger H, Matheis K, Minarik S, Oberle M, Schulz M. TLC - MALDI MS for the Analysis of Lipids. "Planar Chromatography – Mass Spectrometry", Taylor and Francis Verlag, 2015, pp. 213-232, ISBN 9781498705882 .
Eibisch M, Popkova Y, Süß R, Schiller J, Dannenberger D. Evaluation of a commercial enzymatic test kit regarding the quantitative analysis of different free fatty acids. Anal Bioanal Chem. 2014;406:7401-5.
Pirkl A, Meier M, Popkova Y, Letzel M, Schiller J, Schnapp A, Dreisewerd K. Analysis of free fatty acids by ultraviolet laser desorption ionization mass spectrometry from using insect wings as hydrophobic sample substrates. Anal Chem. 2014;86:10763-71.
Griesinger H, Fuchs B, Süß R, Matheis K, Schulz M, Schiller J. The thickness of the stationary phase determines the quality of TLC / MALDI mass spectra of lipids. Anal Biochem. 2014;451:45-7.
Oberbach A, Schlichting N, Neuhaus J, Kullnick Y, Lehmann S, Heinrich M, Dietrich A, Mohr FW, von Bergen M, Baumann S. Establishing a reliable multiple reaction monitoring-based method for the quantification of obesity-associated comorbidities in serum and adipose tissue requires intensive clinical validation. J Proteome Res. 2014;13:5784-800.
Oberbach A, Blüher M, Wirth H, Till H, Kovacs P, Kullnick Y, Schlichting N, Tomm JM, Rolle-Kampczyk U, Murugaiyan J, Binder H, Dietrich A, von Bergen M. Combined proteomic and metabolomic profiling of serum reveals association of the complement system with obesity and identifies novel markers of body fat mass changes. Proteome Res. 2011;10:4769-88.

Doctoral Researcher
Patricia Prabutzki
|
Office address:
|
Room No. R103, Härtelstraße 16-18, 04107 Leipzig
|
|
Phone (lab or office):
E-mail
|
+49 341 97-15722
|
|
PhD project description:
|
Obesity is characterized by the accumulation of excessive body fat predominantly in the form of lipid droplets in adipose tissue (AT) and the detrimental effects that may be associated with factors that can either be secreted from AT or stem from other organs which subsequently affect AT. Spectroscopic and mass spectrometric studies of the lipid composition of different tissue compartments or suitable cell models are therefore necessary to obtain detailed insights into the negative aspects of excessive AT (obesity) and its implications on distinct metabolic pathways. My studies also focus on the role of oxidized lipids and lipid-protein adducts in the pathogenesis of oxidative stress-related diseases, such as type 2 diabetes mellitus.
|
Doctoral Researcher
Alix Aldehoff
|
Office address:
|
Room No. 522, Building 6, Helmholtz-Centre for Environmental Research UFZ, Permoserstraße 15, 04318 Leipzig
|
|
Phone (lab or office):
E-mail
|
+49 341 235-1541
|
|
PhD project description:
|
Alix obtained a Bachelor's degree in Biological Sciences and a Master's degree in Biotechnology from the University of Münster. During her studies, she focused on protein biochemistry, proteomics and metabolomics as tools to investigate functional alterations in intestinal communities caused by environmental pollutants. After finishing her Master's thesis at the Helmholtz-Centre for Environmental Research UFZ in Leipzig, she continued her academic education there as a PhD student. Her PhD project is focusing on lysine acetylation as important post-translational modification impacting various cellular processes, among them adipocyte differentiation. The aim of the project is to investigate lysine acetylation profiles in differentiating adipocytes using mass spectrometry-based methods to get insights into the status of this dynamic modification during exposure to environmental contaminants.
|