A9 - Obesity-driven microglial activation – functional role of cerebral sterol metabolism
Phytosterols are exclusively derived from plant food. Our previous work found that phytosterol (PS) accumulation in the brain is reversible under an obesity-related high fat diet. PS concentrations are in vivo and in vitro inversely correlated with COX-2 and microglia activation. In the next funding period, we want to understand how phytosterols and fatty acids cross the Blood-Brain Barrier (BBB) from circulation and how phytosterols prevent inflammatoric activation of microglia. Phytosterol and fatty acid transport into the brain will be investigated using established in vitro BBB models. The cellular inflammatory response will be investigated in adipose and brain tissue of established mouse and human microglia models and isolated lipid rafts.
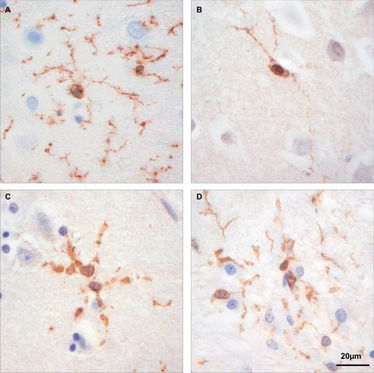
Figure 1. Iba-1 staining shows microglial morphology at different stages of Alzheimer’s disease [Braak&Braak I in (C) and Braak&Braak VI in (D)], in the non-demented old brain (B) and the young brain (A) in representative pictures taken from the hippocampal region. Normal microglia with thin and ramified branches can be seen in the young brain (A), whereas the older individual shows a loss of branches and ramification (B). In AD, microglial cells exhibit spheroid formation and a shortening of branches (C, D). From Tischer et al. (Glia 2016).
Kulow C, Reske A, Leimert M, Bechmann I, Winter K, Steinke H. Topography and evidence of a separate "fascia plate" for the femoral nerve inside the iliopsoas - A dorsal approach. J Anat. 2020 Dec 25.
Hartmann H, Pauli KL, Janssen KL, Huhn S, Ceglarek U, Horstmann A. Preliminary evidence for an association between intake of high‐fat high‐sugar diet, variations in peripheral dopamine precursor availability and dopamine‐dependent cognition in humans. J Neuroendocrinol. 2020 Nov 3:e12917.
Sela I, Yaskolka Meir A, Brandis A, Krajmalnik-Brown R, Zeibich L, Chang D, Dirks B, Tsaban G, Kaplan A, Rinott E, Zelicha H, Arinos S, Ceglarek U, Isermann B, Lapidot M, Green R, Shai I. Wolffia globosa-Mankai Plant-Based Protein Contains Bioactive Vitamin B 12 and Is Well Absorbed in Humans. Nutrients . 2020 Oct 8;12(10):3067.
Piotrowska A, Winter K, Carare RO, Bechmann I. Vital Functions Contribute to the Spread of Extracellular Fluids in the Brain: Comparison Between Life and Death. Front Aging Neurosci. 2020 Feb 11;12:15.
Beuchel C, Becker S, Dittrich J, Kirsten H, Toenjes A, Stumvoll M, Loeffler M, Thiele H, Beutner F, Thiery J, Ceglarek U, Scholz M., Clinical and lifestyle related factors influencing whole blood metabolite levels - A comparative analysis of three large cohorts. Mol Metab. 2019 Nov;29:76-85.
Zelicha H, Kaplan A, Yaskolka Meir A, Tsaban G, Rinott E, Shelef I, Tirosh A, Brikner D, Pupkin E, Qi L, Thiery J, Stumvoll M, Kloting N, von Bergen M, Ceglarek U, Blüher M, Stampfer MJ, Shai I. The Effect of Wolffia globosa Mankai, a Green Aquatic Plant, on Postprandial Glycemic Response: A Randomized Crossover Controlled Trial. Diabetes Care. 2019 Jul;42(7):1162-1169
García-Cáceres C, Balland E, Prevot V, Luquet S, Woods SC, Koch M, Horvath TL, Yi CX, Chowen JA, Verkhratsky A, Araque A, Bechmann I, Tschöp MH. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat Neurosci. 2019 Jan;22(1):7-14.
Joost E, Jordão MJC, Mages B, Prinz M, Bechmann I, Krueger M. Microglia contribute to the glia limitans around arteries, capillaries and veins under physiological conditions, in a model of neuroinflammation and in human brain tissue. Brain Struct Funct. 2019 Jan 31.
Wagner R, Dittrich J, Thiery J, Ceglarek U, Burkhardt R. Simultaneous LC-MS/MS quantification of eight apolipoproteins in normal and hypercholesterolemic mouse plasma. J Lipid Res. 2019 Feb 5. pii: jlr.D084301
Dittrich J, Beutner F, Teren A, Thiery J, Burkhardt R, Scholz M, Ceglarek U. Plasma levels of apolipoproteins C-III, A-IV, and E are independently associated with stable atherosclerotic cardiovascular disease. Atherosclerosis. 2018 Nov 9;281:17-24.
Reinicke M, Schröter J, lMüller-Klieser D,Helmschrodt C, Ceglarek U. Free oxysterols and bile acids including conjugates - Simultaneous quantification in human plasma and cerebrospinal fluid by liquid chromatography-tandem mass spectrometry. Analytica Chimica Acta, march 2018
Kälin S, Heppner FL, Bechmann I, Prinz M, Tschöp MH, Yi CX. Hypothalamic innate immune reaction in obesity. Nat Rev Endocrinol. 2015;11:339-51.
Koch M, Varela L, Kim JG, Kim JD, Hernandez-Nuno F, Simonds SE, Castorena CM, Vianna CR, Elmquist JK, Morozov YM, Rakic P, Bechmann I, Cowley MA, Szigeti-Buch K, Dietrich MO, Gao XB, Diano S, Horvath TL. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519:45-50.
Yi CX, Gericke M, Krüger M, Alkemade A, Kabra DG, Hanske S, Filosa J, Pfluger P, Bingham N, Woods SC, Herman J, Kalsbeek A, Baumann M, Lang R, Stern JE, Bechmann I, Tschöp MH. High calorie diet triggers hypothalamic angiopathy. Mol Metab. 2012;1:95-100.
Burkhardt R, Kirsten H, Beutner F, Holdt M, Gross A, Teren A, Tönjes A, Becker S, Krohn K, Kovacs P, Stumvoll M, Teupser D, Thiery J, Ceglarek U, Scholz M. Integration of genome-wide SNP data and gene-expression profiles reveals six novel loci and regulatory mechanisms for amino acids and acylcarnitines in whole blood. PLoS Genet. 2015;11:e1005510.
Helmschrodt C, Becker S, Thiery J, Ceglarek U. Preanalytical standardization for reactive oxygen species derived oxysterol analysis in human plasma by liquid chromatography-tandem mass spectrometry. Biochem Biophys Res Commun. 2014;446:726-30.
Kortz L, Dorow J, Ceglarek U. Liquid chromatography-tandem mass spectrometry for the analysis of eicosanoids and related lipids in human biological matrices: a review. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;964:1-11.
Ceglarek U, Dittrich J, Becker S, Baumann F, Kortz L, Thiery J. Quantification of seven apolipoproteins in human plasma by proteotypic peptides using fast LC-MS/MS. Proteomics Clin Appl. 2013;11-12:794-801.
Brauer R, Leichtle AB, Fiedler GM, Thiery J, Ceglarek U. Preanalytical standardization of amino acid and acylcarnitine metabolite profiling in human blood using tandem mass spectrometry. Metabolomics. 2011;7:344-52.
Prodinger C, Bunse J, Krüger M, Schiefenhövel F, Brandt C, Laman JD, Greter M, Immig K, Heppner F, Becher B, Bechmann I. CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system. Acta Neuropathol. 2011;121:445-58.
Streit WJ, Braak H, Xue QS, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta Neuropathol. 2009;118:475-85.
Postdoc
Madlen Reinicke
| Office address: | Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics, Paul-List-Str. 13-15, 04103 Leipzig |
| Phone (lab or office):E-mail: |
+49 341 / 97 22481
|


Doctoral Researcher
Judith Leyh
| Office address: | Faculty of Medicine, Institute of Anatomy, Liebigstraße 13, 04103 Leipzig |
|
Phone (lab or office):
E-mail:
|
+49 341 97 22007
|
| Research subject: |
Microglia
|
Doctoral Researcher
Laura Plantera
| Office address: | Faculty of Medicine, Institute of Anatomy, Liebigstraße 13, 04103 Leipzig |
|
Phone (lab or office):
E-mail:
|
+49 341 97 22007
|
| Research subject: |
Microglia
|