B6 - Mechano-sensitive adhesion GPCR in adipose tissue
We will test the hypothesis that mechano-activation of adhesion GPCRs (aGPCR) regulates adipocyte differentiation and metabolism in vivo. Thus, we will analyze i) signaling pathways modulated by distinct aGPCRs under mechanical force application, ii) the functional impact of mechano-stimulation on adipocyte function, and iii) the in vivo relevance of mechano-sensitive aGPCR in adipose tissue (AT) of mice and humans. This will be achieved using live imaging, atomic force microscopy and animal models of aGPCR deficiency. In a translational step, function-altering variants of aGPCRs in humans will then be clinically examined in the framework of this CRC and explored as a potential therapeutic target in obese patients.
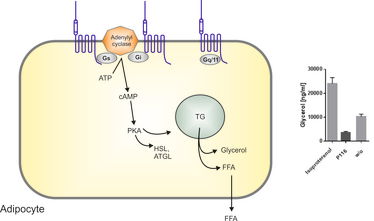
Figure 1: Adhesion G protein-coupled receptors (aGPCRs) modulate adipocyte function. Several members of the aGPCR family have been identified to be differentially regulated in adipocytes and adipose tissue. We propose that aGPCRs can modify adipocyte function and fatty acid composition through G protein-mediated functions in vitro and in vivo.
PKA: protein kinase A, HSL: hormone-sensitive lipase, ATGL: adipose triglyceride lipase, TG: triglyceride, FFA: free fatty acid, p116: agonistic peptide of GPR116 (an aGPCR that is endogenously expressed in adipose tissue), w/o: without.
Kaczmarek I, Suchý T, Prömel S, Schöneberg T, Liebscher I, Thor D. The relevance of adhesion G protein-coupled receptors in metabolic functions.Biol Chem. 2021 Jul 5.
Frenster JD, Stephan G, Ravn-Boess N, Bready D, Wilcox J, Kieslich B, Wilde C, Sträter N, Wiggin GR, Liebscher I, Schöneberg T, Placantonakis DG. Functional impact of intramolecular cleavage and dissociation of adhesion G protein-coupled receptor GPR133 (ADGRD1) on canonical signaling. J Biol Chem. 2021 May 19:100798.
Georgiadi A, Lopez-Salazar V, Merahbi RE, Karikari RA, Ma X, Mourão A, Klepac K, Bühler L, Alfaro AJ, Kaczmarek I, Linford A, Bosma M, Shilkova O, Ritvos O, Nakamura N, Hirose S, Lassi M, Teperino R, Machado J, Scheideler M, Dietrich A, Geerlof A, Feuchtinger A, Blutke A, Fischer K, Müller TD, Kessler K, Schöneberg T, Thor D, Hornemann S, Kruse M, Nawroth P, Pivovarova-Ramich O, Pfeiffer AFH, Sattler M, Blüher M, Herzig S. Orphan GPR116 mediates the insulin sensitizing effects of the hepatokine FNDC4 in adipose tissue. Nat Commun. 2021 May 20;12(1):2999.
Favara DM, Liebscher I, Jazayeri A, Nambiar M, Sheldon H, Banham AH, Harris AL. Elevated expression of the adhesion GPCR ADGRL4/ELTD1 promotes endothelial sprouting angiogenesis without activating canonical GPCR signalling. Sci Rep. 2021 Apr 23;11(1):8870.
Schöneberg T, Liebscher I. Mutations in G Protein-Coupled Receptors: Mechanisms, Pathophysiology and Potential Therapeutic Approaches. Pharmacol Rev. 2021 Jan;73(1):89-119.
Le Duc D, Lin CC, Popkova Y, Yang Z, Akhil V, Çakir MV, Grunewald S, Simon JC, Dietz A, Dannenberger D, Garten A, Lemke JR, Schiller J, Blüher M, Nono Nankam PA, Rolle-Kampczyk U, von Bergen M, Kelso J, Schöneberg T. Reduced lipolysis in lipoma phenocopies lipid accumulation in obesity. Int J Obes (Lond). 2020 Nov 24.
Jäger E, Murthy S, Schmidt C, Hahn M, Strobel S, Peters A, Stäubert C, Sungur P, Venus T, Geisler M, Radusheva V, Raps S, Rothe K, Scholz R, Jung S, Wagner S, Pierer M, Seifert O, Chang W, Estrela-Lopis I, Raulien N, Krohn K, Sträter N, Hoeppener S, Schöneberg T, Rossol M, Wagner U. Calcium-sensing receptor-mediated NLRP3 inflammasome response to calciprotein particles drives inflammation in rheumatoid arthritis. Nat Commun. 2020 Aug 25;11(1):4243.
Frenster JD, Kader M, Kamen S, Sun J, Chiriboga L, Serrano J, Bready D, Golub D, Ravn-Boess N, Stephan G, Chi AS, Kurz SC, Jain R, Park CY, Fenyo D, Liebscher I, Schöneberg T, Wiggin G, Newman R, Barnes M, Dickson JK, MacNeil DJ, Huang X, Shohdy N, Snuderl M, Zagzag D, Placantonakis DG. Expression profiling of the adhesion G protein-coupled receptor GPR133 (ADGRD1) in glioma subtypes. Neurooncol Adv. 2020 Apr 28;2(1):vdaa053.
Suchý T, Zieschang C, Popkova Y, Kaczmarek I, Weiner J, Liebing AD, Çakir MV, Landgraf K, Gericke M, Pospisilik JA, Körner A, Heiker JT, Dannenberger D, Schiller J, Schöneberg T, Liebscher I, Thor D. The repertoire of Adhesion G protein-coupled receptors in adipocytes and their functional relevance. Int J Obes (Lond). 2020 Mar 19.
Peters A, Rabe P, Krumbholz P, Kalwa H, Kraft R, Schöneberg T, Stäubert C. Natural biased signaling of hydroxycarboxylic acid receptor 3 and G protein-coupled receptor 84. Cell Commun Signal. 2020 Feb 26;18(1):31.
Röthe J, Kraft R, Schöneberg T, Thor D. Exploring G Protein-Coupled Receptor Signaling in Primary Pancreatic Islets. Biol Proced Online. 2020 Feb 15;22:4.
Leppert B, Strunz S, Seiwert B, Schlittenbauer L, Schlichting R, Pfeiffer C, Röder S, Bauer M, Borte M, Stangl GI, Schöneberg T, Schulz A, Karkossa I, Rolle-Kampczyk UE, Thürmann L, von Bergen M, Escher BI, Junge KM, Reemtsma T, Lehmann I, Polte T. Maternal paraben exposure triggers childhood overweight development. Nat Commun. 2020 Feb 11;11(1):561.
Jacobson KA, Delicado EG, Gachet C, Kennedy C, von Kügelgen I, Li B, Miras-Portugal MT, Novak I, Schöneberg T, Perez-Sen R, Thor D, Wu B, Yang Z, Müller CE. Update of P2Y Receptor Pharmacology: IUPHAR Review:27. Br J Pharmacol. 2020 Feb 9.
Bradley EC, Cunningham RL, Wilde C, Morgan RK, Klug EA, Letcher SM, Schöneberg T, Monk KR, Liebscher I, Petersen SC. In vivo identification of small molecules mediating Gpr126/Adgrg6 signaling during Schwann cell development., Ann N Y Acad Sci. 2019 Sep 16.
Eichler W, Lohrenz A, Simon KU, Krohn S, Lange J, Bürger S, Liebscher I. The role of ADGRE5/CD97 in human retinal pigment epithelial cell growth and survival. Ann N Y Acad Sci. 2019 Aug 9.
Scholz N, Langenhan T, Schöneberg T. Revisiting the classification of adhesion GPCRs. Ann N Y Acad Sci. 2019 Jul 31.
Knierim AB, Röthe J, Çakir MV, Lede V, Wilde C, Liebscher I, Thor D, Schöneberg T. Genetic basis of functional variability in adhesion G protein-coupled receptors. Sci Rep. 2019 Jul 30;9(1):11036
Röthe J, Thor D, Winkler J, Knierim AB, Binder C, Huth S, Kraft R, Rothemund S, Schöneberg T, Prömel S. Involvement of the Adhesion GPCRs Latrophilins in the Regulation of Insulin Release. Cell Rep. 2019 Feb 5;26(6):1573-1584.
Schöneberg T, Prömel S. Latrophilins and Teneurins in Invertebrates: No Love for Each Other? Front. Neurosci., 12 March 2019
Schöneberg T, Meister J, Knierim AB, Schulz A. The G protein-coupled receptor GPR34 - The past 20 years of a grownup. Pharmacol Ther. 2018 Apr 22. pii: S0163-7258(18)30071-8.
Lede V, Meusel A, Garten A, Popkova Y, Penke M, Franke C, Ricken A, Schulz A, Kiess W, Huster D, Schöneberg T, Schiller J. Altered hepatic lipid metabolism in mice lacking both the melanocortin type 4 receptor and low density lipoprotein receptor. PLoS One. 2017;12:e0172000.
Lede V, Franke C, Meusel A, Teupser D, Ricken A, Thiery J, Schiller J, Huster D, Schöneberg T, Schulz A. Severe atherosclerosis and hypercholesterolemia in mice lacking both the melanocortin type 4 receptor and low density lipoprotein receptor. PLoS One. 2016;11:e0167888.
Sträter N, Marek S, Kuettner EB, Kloos M, Keim A, Brüser A, Kirchberger J, Schöneberg T. Molecular architecture and structural basis of allosteric regulation of eukaryotic phosphofructokinases. FASEB J. 2011;25:89-98.
Bohnekamp J, Schöneberg T. Cell adhesion receptor GPR133 couples to Gs protein. J Biol Chem. 2011; 286: 41912-6.
Engel KMY, Schröck K, Teupser D, Holdt LM, Tönjes A, Kern M, Dietrich K, Kovacs P, Krügel U, Scheidt HA, Schiller J, Huster D, Brockmann GA, Augustin M, Thiery J, Blüher M, Stumvoll M, Schöneberg T, Schulz A. Reduced food intake and body weight in mice deficient for the G protein-coupled receptor GPR82. PLoS One. 2011;6:e29400.
Stäubert C, Tarnow P, Brumm H, Pitra C, Gudermann T, Grüters A, Schöneberg T, Biebermann H, Römpler H. Evolutionary aspects in evaluating mutations in the melanocortin 4 receptor. Endocrinology. 2007;148:4642-8.
Doctoral Researcher
Isabell Kaczmarek
|
Office address:
|
Room No. D306, Rudolf Schoenheimer Institute of Biochemistry, Johannisallee 30, 04103 Leipzig
|
|
Phone (lab or office):
E-mail
|
+49 341 9722177
|
|
PhD project description:
|
Isabell is a PhD student in the group of Dr. Doreen Thor at Rudolf-Schönheimer-Institute. Here, she works on the deorphanization of adhesion G protein-coupled receptors (aGPCR) regulated in adipose tissue of obese compared to lean individuals. Therefore, she establishes cell models for hypertrophy, metabolically characterizes primary cells and cell cultures after artificial activation or knockdown of aGPCR and analyzes RNAseq dataset.
|


Doctoral Researcher
Christina Kuhn
|
Office address:
|
Rudolf Schoenheimer Institute of Biochemistry, Johannisallee 30, 04103 Leipzig
|
|
Phone (lab or office):
E-mail
|
+49 341 9722152
|
|
PhD project description:
|
Christina is a PhD student in the group of Dr. Susanne Horn at Rudolf-Schönheimer-Institute. Here, she develops machine-learning models in python for the prediction of clinical outcome from high throughput sequencing data. These are applied to decipher the characteristics of melanoma tumor samples during immune checkpoint inhibition.
|