B9 - Role of macrophages in adipocyte degradation – a live-imaging approach
The innate immune system is involved in tissue homeostasis due to the clearance of dead cells and tissue regeneration. Every day 200 – 300 billion dead cells have to be recognized and degraded by specialized phagocytes, so-called macrophages. In contrast to other tissues, adipose tissue mainly consists of fat cells (adipocytes), exceeding 100 µm in diameter, a dimension about 5~fold larger than regular macrophages. Hence, tissue clearing of dead adipocytes is challenging. Our preliminary data show that single macrophages are insufficient to degrade dead adipocytes. This project aims to elucidate the cellular and molecular processes following adipocyte death. Therefore, we have established ex vivo models (laser-injury of individual adipocytes) and in vivo models (transgenic expression of cell death inducers) to understand the cellular and molecular pathways leading to adipocyte degradation in living tissue. We will here focus on the extracellular digestion of adipocytes via lysosomal exocytosis. Our ultimate goal is to provide pharmacological targets to improve adipocyte degradation of dead adipocytes in obesity.
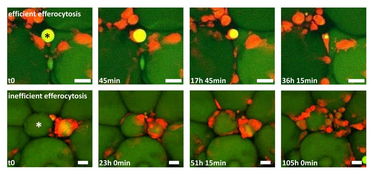
Figure 1: Two time-lapse movies unravel fundamentally different clearing behaviour of macrophages in adipose tissue (AT). AT explants showing macrophages (red) and adipocytes (green). Upper row: One macrophage detects, internalizes and digests a small adipocyte remnant (star), a process leading to efficient removal of the adipocyte remnant within ~36 h. Lower row: After initial contact between macrophages and a hypertrophic adipocyte (star), more macrophages become attracted, resulting in a CLS formation with now signs of efficient degradation after 4 days. Scale bar represents 50 µm.
Krieg L, Didt K, Karkossa I, Bernhart SH, Kehr S, Subramanian N, Lindhorst A, Schaudinn A, Tabei S, Keller M, Stumvoll M, Dietrich A, von Bergen M, Stadler PF, Laurencikiene J, Krüger M, Blüher M, Gericke M, Schubert K, Kovacs P, Chakaroun R, Massier L. Gut. 2021 Oct 1:gutjnl-2021-324603.
Lindhorst A, Raulien N, Wieghofer P, Eilers J, Rossi FMV, Bechmann I, Gericke M. Adipocyte death triggers a pro-inflammatory response and induces metabolic activation of resident macrophages. Cell Death Dis . 2021 Jun 5;12(6):579.
Brinker G, Froeba J, Arndt L, Braune J, Hobusch C, Lindhorst A, Bechmann I, Gericke M. CD4+ T cells regulate glucose homeostasis independent of adipose tissue dysfunction in mice. Eur J Immunol. 2021 Mar 30.
Kunath A, Weiner J, Krause K, Rehders M, Pejkovska A, Gericke M, Biniossek ML, Dommel S, Kern M, Ribas-Latre A, Schilling O, Brix K, Stumvoll M, Klöting N, Heiker JT, Blüher M. Role of Kallikrein 7 in Body Weight and Fat Mass Regulation. Biomedicines 2021, 9, 131.
Landgraf K, Klöting N, Gericke M, Maixner N, Guiu-Jurado E, Scholz M, Witte AV, Beyer F, Schwartze JT, Lacher M, Villringer A, Kovacs P, Rudich A, Blüher M, Kiess W, Körner A.The Obesity-Susceptibility Gene TMEM18 Promotes Adipogenesis through Activation of PPARG. Cell Rep. 2020;33(3):108295.
Lindhorst A, Bechmann I, Gericke M. Unspecific DNA recombination in AdipoqCre-ERT2 – mediated knockout approaches in transgenic mice is sex-, age- and genotype-dependent. Adipocyte 2020 Dec;9(1):1-6.
Braune J, Lindhorst A, Fröba J, Hobusch C, Kovacs P, Blüher M, Eilers J, Bechmann I, Gericke M. Multinucleated Giant Cells in Adipose Tissue are Specialized in Adipocyte Degradation. Diabetes. 2020:db200293.
Schleinitz D, Krause K, Wohland T, Gebhardt C, Linder N, Stumvoll M, Blüher M, Bechmann I, Kovacs P, Gericke M, Tönjes A. Identification of distinct transcriptome signatures of human adipose tissue from fifteen Depots. Eur J Hum Genet. 2020 Jul 13.
Dommel S, Berger C, Kunath A, Kern M, Gericke M, Kovacs P, Guiu-Jurado E, Klöting N, Blüher M. The Fabp4-Cre-model is insufficient to study Hoxc9 function in adipose tissue. Biomedicines . 2020 Jun 29;8(7):E184.
Schopow N, Kallendrusch S, Gong S, Rapp F, Körfer J, Gericke M, Spindler N, Josten C, Langer S, Bechmann I. Examination of Ex-Vivo Viability of Human Adipose Tissue Slice Culture. PLoS One. 2020 May 26;15(5):e0233152.
Massier L, Chakaroun R, Tabei S, Crane A, Didt KD, Fallmann J, von Bergen M, Haange SB, Heyne H, Stumvoll M, Gericke M, Dietrich A, Blüher M, Musat N, Kovacs P. Adipose tissue derived bacteria are associated with inflammation in obesity and type 2 diabetes. Gut. 2020 Apr 21. pii: gutjnl-2019-320118.
Piotrowska A, Winter K, Carare RO, Bechmann I. Vital Functions Contribute to the Spread of Extracellular Fluids in the Brain: Comparison Between Life and Death. Front Aging Neurosci. 2020 Feb 11;12:15.
Zhao S, Todorov MI, Cai R, -Maskari RA, Steinke H, Kemter E, Mai H, Rong Z, Warmer M, Stanic K, Schoppe O, Paetzold JC, Gesierich B, Wong MN, Huber TB, Duering M, Bruns OT, Menze B, Lipfert J, Puelles VG, Wolf E, Bechmann I, Ertürk A. Cellular and Molecular Probing of Intact Human Organs. Cell. 2020 Feb 20;180(4):796-812.e19.
Streit WJ, Khoshbouei H, Bechmann I. Dystrophic microglia in late-onset Alzheimer's disease. Glia. 2020 Apr;68(4):845-854.
Hadadi-Bechor S, Haim Y, Pecht T, Gat R, Tarnovsky T, Gericke M, Rudich A. Autophagy differentially regulates macrophage lipid handling depending on the lipid substrate (oleic acid vs. acetylated-LDL) and inflammatory activation state. Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Sep 11:158527
García-Cáceres C, Balland E, Prevot V, Luquet S, Woods SC, Koch M, Horvath TL, Yi CX, Chowen JA, Verkhratsky A, Araque A, Bechmann I, Tschöp MH. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat Neurosci. 2019 Jan;22(1):7-14.
Joost E, Jordão MJC, Mages B, Prinz M, Bechmann I, Krueger M. Microglia contribute to the glia limitans around arteries, capillaries and veins under physiological conditions, in a model of neuroinflammation and in human brain tissue. Brain Struct Funct. 2019 Jan 31.
Streit WJ, Braak H, Del Tredici K, Leyh J, Lier J, Khoshbouei H, Eisenlöffel C, Müller W, Bechmann I. Microglial activation occurs late during preclinical Alzheimer's disease. Glia. 2018 Nov 11.
Haimon Z, Volaski A, Orthgiess J, Boura-Halfon S, Varol D, Shemer A, Yona S, Zuckerman B, David E, Chappell-Maor L, Bechmann I, Gericke M, Ulitsky I, Jung S., Re-evaluating microglia expression profiles using RiboTag and cell isolation strategies. Nat Immunol. 2018 May 18.
Zieger K, Weiner J, Kunath A, Gericke M, Krause K, Kern M, Stumvoll M, Klöting N, Blüher M, Heiker JT. Ablation of kallikrein 7 (KLK7) in adipose tissue ameliorates metabolic consequences of high-fat diet induced obesity by counteracting adipose tissue inflammation in vivo. Cell Mol Life Sci. 2017; Epub ahead of print.
Braune J, Weyer U, Hobusch C, Mauer J, Brüning J, Bechmann I, Gericke M. IL-6 regulates M2 polarization and local proliferation of adipose tissue macrophes in obesity. J Immunol. 2017;198:2927-34.
Braune J, Weyer U, Matz-Soja M, Hobusch C, Kern M, Kunath A, Klöting N, Kralisch S, Blüher M, Gebhardt R, Zavros Y, Bechmann I, Gericke M. Hedgehog sinaling in myeloid cells impacts on body weight, adipose tissue inflammation and glucose metabolism. Diabetologia. 2017;60:889-99.
Orthgiess J, Gericke M, Immig K, Schulz A, Hirrlinger J, Bechmann I, Eilers J. Neurons exhibit Lyz2 promotor activity in vivo: Implications for using LysM-Cre mice in myeloid cell research. Eur J Immunol. 2016;46:1529-32.
Haim Y, Blüher M, Slutsky N, Goldstein N, Klöting N, Harman-Boehm I, Kirshstein B, Ginsberg D, Gericke M, Guiu Jurado E, Kovsan J, Tarnovscki T, Kachko L, Bashan N, Gepner Y, Shai I, Rudich A. Elevated autophagy gene expression in adipose tissue of humans: A potential non-cell-cycle-dependent function of E2F1. Autophagy. 2015;11:2074-88.
du Plessis J, van Pelt J, Korf H, Mathieu C, van der Schueren B, Lannoo M, Oyen T, Topal B, Fetter G, Nayler S, van der Merwe T, Windmolders P, van Gaal L, Verrijken A, Hubens G, Gericke M, Cassiman D, Francque S, Nevens F, van der Merwe S. Association of adipose tissue inflammation with histological severity of non-alcoholic fatty liver disease. Gastroenterology. 2015;149:635-48.
Gericke M, Weyer U, Braune J, Bechmann I, Eilers J. A method for long-term live-imaging of tissue macrophages in adipose tissue explants. Am J Physiol Endocrinol Metab. 2015;308:E1023-33.
Kosacka J, Kern M, Klöting N, Paeschke S, Rudich A, Haim Y, Gericke M, Serke H, Stumvoll M, Bechmann I, Nowicki M, Blüher M. Autophagy in adipose tissue of patients with obesity and type 2 diabetes. Mol Cell Endocrinol. 2015;409:21-32.
Immig K, Gericke M, Menzel F, Merz F, Krueger M, Schiefenhövel F, Lösche A, Jäger K, Hanisch UK, Biber K, Bechmann I. CD11c-positive cells from brain, spleen, lung and liver exhibit site-specific immune phenotypes and plastically adapt to new environments. Glia. 2015;63:11-25.
Haase J, Weyer U, Immig K, Klöting N, Blüher M, Eilers J, Bechmann I, Gericke M. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia. 2014;57:562-71.
Gericke M, Schröder T, Kosacka J, Nowicki M, Klöting N, Spanel-Borowski K. Neuropeptide Y impairs insulin-stimulated translocation of glucose transporter 4 in 3T3-L1 adipocytes through the Y1 receptor. Mol Cell Endocrinol. 2012;348:27-32.
Prodinger C, Bunse J, Krüger M, Schiefenhövel F, Brandt C, Laman JD, Greter M, Immig K, Heppner F, Becher B, Bechmann I. CD11c-expressing cells reside in juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system. Acta Neuropathol. 2011;121:445-58.
Gericke M, Kosacka J, Koch D, Nowicki M, Schröder T, Ricken AM, Nieber K, Spanel-Borowski K. Receptors for NPY and PACAP differ in expression and activity during adipogenesis in the murine 3T3-L1 fibroblast cell line. Br J Pharmacol. 2009;157:620-32.
Doctoral Researcher
Andreas Lindhorst
|
Office address:
|
Room No. 103, Liebigstr.13, 04103 Leipzig
|
|
Phone (lab or office):
E-mail
|
+49 341 9722053
|
|
PhD project description:
|
Adipocyte size exceeds the threshold for classical, anti-inflammatory efferocytosis by macrophages. Instead, lysosomal exocytosis is proposed as degradation pathway of dead adipocytes. We developed a new model to induce adipocyte death in a live imaging approach that allows to track initial adipocyte - macrophage interaction and to analyze phenotypical changes in macrophages following adipocyte death. Our goal is to elucidate the mechanistics behind the extracellular degestion of adipocytes and identify pharmacological targets to improve degradation of dead adipocytes in obesity.
|


Doctoral Researcher
Raphaela Wehr
|
Office address:
|
Room 104, Liebigstr. 13, 04103 Leipzig |
|
Phone (lab or office):
E-mail
|
+49 341 9722007
raphaela.wehr@medizin.uni-leipzig.de |
|
PhD project description:
|
Raphaela is the new doctoral researcher in the Group of Martin Gericke on the institute of anatomy. She will continue the research on the mechanisms of lysosomal exocytosis of dead adipocytes by macrophages. She is investigating calcium-dependent processes of this extracellular degestion of adipocytes. The goal is to identify possible pharmacological targets to reduce the inflammation of adipose tissue in obesity, caused by dead adipocytes.
|