Hepatic lipid accumulation in mouse models of obesity
This project has been funded by the DFG from 2013 to 2016.
Project description of the 1st funding period:
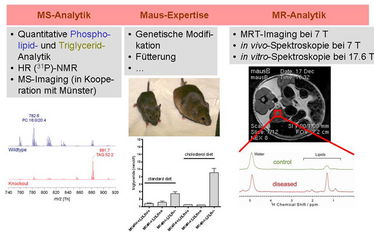
Obesity is accompanied by insulin resistance, hypertension and dyslipidemia what is referred to as the "metabolic syndrome". Dyslipidemia and hepatosteatosis are also frequent consequences of obesity. We have previously established a genetic mouse strain deficient for the hypothalamic melanocortin type 4 receptor (MC4R) and the low-density lipoprotein (LDL) receptor that exhibits both, metabolic syndrome and hyperlipidemia with a lipoprotein (LP) profile similar to that in obese humans. Within six months these double-mutant mice develop hepatosteatosis and atherosclerosis already under standard diet, not containing significant levels of cholesterol. This mouse model will be used to study mechanisms leading to consequences of obesity, specifically the changes in hepatic lipid metabolism. A central question of this project is if there are molecular differences in the hepatic transcriptome and lipid metabolome and, therefore, pathomechanisms depending on the cause of obesity-associated hepatosteatosis. LDLR/ and double LDLR/MC4R-deficient mice as well as other murine obesity models will be investigated in dependence on the diet. Profiling the changes in the hepatic transcriptome and lipid metabolome will be performed by RNA sequencing and mass spectrometry (detection of the individual lipid classes and their fatty acyl compositions) as well as NMR spectroscopy under high resolution (HR) and magic-angle spinning (MAS) conditions in order to determine the water and lipid contents as well as the individual lipid species. We will extract and attempt to understand the initial signals and metabolic consequences leading to hepatitis and hepatosteatosis in mouse models and also in obese humans. It is particularly expected that the applied analytical methods and comparisons of different obese animal models will help to evaluate the roles of lipid oxidation products and obesity model-specific changes which may have implications in pathogenesis and clinical prognosis of hepatosteatosis. While the analyses will be primarily carried out on biopsies, we will also utilize magnetic resonance imaging techniques at 7 Tesla to analyze fat tissues and the liver in vivo with spatial resolutions.
Figure 1. Model of dysfunction in development of fat tissue
Schröter J, Schiller J. Chlorinated phospholipids and fatty acids: (patho)physiological relevance, potential toxicity, and analysis of lipid chlorohydrins. Oxid Med Cell Longev. 2016;2016:8386362.
Lede V, Franke C, Meusel A, Teupser D, Ricken A, Thiery J, Schiller J, Huster D, Schöneberg T, Schulz A. Severe atherosclerosis and hypercholesterolemia in mice lacking both the melanocortin type 4 receptor and low density lipoprotein receptor. PLoS One. 2016;11:e0167888.
Stelzner K, Herbert D, Popkova Y, Lorz A, Schiller J, Gericke M, Klöting N, Blüher M, Franz S, Simon JC, Saalbach A. Free fatty acids sensitize dendritic cells to amplify TH1/TH17-immune responses. Eur J Immunol. 2016;46:2043-53.
Schröter J, Griesinger H, Reuß E, Schulz M, Riemer T, Süß R, Schiller J, Fuchs B. Unexpected products of the hypochlorous acid-induced oxidation of oleic acid: A study using high performance thin-layer chromatography-electrospray ionization mass spectrometry. J Chromatogr A. 2016;1439:89-96.
Popkova Y, Meusel A, Breitfeld J, Schleinitz D, Hirrlinger J, Dannenberger D, Kovacs P, Schiller J. Nutrition-dependent changes of mouse adipose tissue compositions monitored by NMR, MS, and chromatographic methods. Anal Bioanal Chem. 2015;407:5113-23.
Griesinger H, Fuchs B, Süß R, Matheis K, Schulz M, Schiller J. The thicknesses of the stationary phase determines the quality of TLC/MALDI mass spectra of lipids. Anal Biochem. 2014;451:45-7.
Jaskolla TW, Onischke K, Schiller J. 2,5-dihydroxybenzoic acid salts for matrix-assisted laser desorption/ionizatioin time-of-flight mass spectrometric lipid analysis: Simplified spectra interpretation and insights into gasphase fragmentation. Rapid Commun Mass Spetrom. 2014;28:1353-63.
Zschörnig K, Schiller J. A simple method to generate oxidized phosphatidylcholines im amounts close to one milligram. Chem Phys Lipids. 2014;184:30-7.
Eibisch M, Popkova Y, Süß R, Schiller J, Dannenberger D. Evaluation of a commercial enzymatic test kit regarding the quantitative analysis of different free fatty acids. Anal Bioanal Chem. 2014;406:7410-5.
Pirkl A, Meier M, Popkova Y, Letzel M, Schnapp A, Schiller J, Dreisewerd K. Analysis of free fatty acids by ultraviolet laser desorption ionization mass spectrometry using insect wings as hydrophobic sample substrates. Anal Chem. 2014;86:10763-71.
Scheidt HA, Meyer T, Nikolaus J, Baek DJ, Haralampiev I, Thomas L, Bittmann R, Müller P, Herrmann A, Huster D. Cholesterol[1]s Aliphatic Side Chain Modulates Membrane Properties. Angew Chem Int Ed. 2013;52:12848–51.
Weber F, Böhme J, Scheidt HA, Gründer W, Rammelt S, Hacker M, Schulz-Siegmund M, Huster D. 31P and 13C solid-state NMR spectroscopy to study collagen synthesis and biomineralization in polymer-based bone implants. NMR Biomed. 2012;25:464-75.
Fuchs B, Süß R, Teuber K, Eibisch M, Schiller J. Application of TLC to the analysis of lipids. J Chromatogr A. 2011;1218:2754-74.
Sträter N, Marek S, Kuettner EB, Kloos M, Keim A, Brüser A, Kirchberger J, Schöneberg T. Molecular architecture and structural basis of allosteric regulation of eukaryotic phosphofructokinases. FASEB J. 2011;25:89-98.
Bohnekamp J, Schöneberg T. Cell adhesion receptor GPR133 couples to Gs protein. J Biol Chem. 2011; 286: 41912-6.
Engel KMY, Schröck K, Teupser D, Holdt LM, Tönjes A, Kern M, Dietrich K, Kovacs P, Krügel U, Scheidt HA, Schiller J, Huster D, Brockmann GA, Augustin M, Thiery J, Blüher M, Stumvoll M, Schöneberg T, Schulz A. Reduced food intake and body weight in mice deficient for the G protein-coupled receptor GPR82. PLoS One. 2011;6:e29400.
Fuchs B, Süß R, Schiller J. An update of MALDI-TOF mass spectrometry in lipid research. Progr Lipid Res. 2010;49:450-75.
Jaskolla T, Fuchs B, Karas M, Schiller J. The new matrix 4-chloro-α-cyanocinnamic acid allows the detection of phosphatidylethanolamine chloramines by MALDI-TOF mass spectrometry. J Am Soc Mass Spectrom. 2009;20:867-74.
Richter G, Schober C, Süß R, Fuchs B, Birkemeyer C, Schiller J. Comparison of the positive and negative ion electrospray ionization and matrix-assisted laser desorption and ionization time-of-flight mass spectra of the reaction products of phosphatidylethanolamines and hypochlorous acid. Anal Biochem. 2008;376:157-9.
Bunge A, Müller P, Stöckl M, Herrmann A, Huster D. Characterization of the ternary mixture of sphingomyelin, POPC, and cholesterol. Support for an inhomogeneous lipid distribution at high temperature. Biophys J. 2008;94:2680-90.
Stäubert C, Tarnow P, Brumm H, Pitra C, Gudermann T, Grüters A, Schöneberg T, Biebermann H, Römpler H. Evolutionary aspects in evaluating mutations in the melanocortin 4 receptor. Endocrinology. 2007;148:4642-8.
Huster D, Scheidt HA, Arnold K, Herrmann A, Müller P. Desmosterol may replace cholesterol in biological membranes. Biophys J. 2005;88:1838-44.
Huster D, Arnold K, Gawrisch K. Influence of docosahexaenoic acid and cholesterol on lateral lipid organization in phospholipid membranes. Biochemistry. 1998;37:17299-308.
PROJECT TEAM
|
Prof. Dr. Torsten Schöneberg |
PD Dr. Jürgen Schiller |
Prof. Dr. Daniel Huster |