Z5 - Molecular mechanisms of action of adipokines
Adipokines are released from adipose tissue and influence the metabolism of the whole organism. Accordingly, increased body fat leads to an increased or reduced release of adipokines dependent on their regulation which can subsequently explain dysfunctions in obesity. Several adipokines have been correlated with obesity, including adiponectin, vaspin, Dlk1/Pref-1 (delta-like homolog 1; preadipocyte factor 1) and chemerin. However, so far little is known about their molecular mechanism of function. We successfully cloned and expressed full-length and globular adiponectin, vaspin, Dlk1/Pref-1, prochemerin and chemerin. These adipokines have been identified by genetic studies or expression analysis of visceral and subcutaneous fat. The aim of the project is now to identify their targets, to find novel interacting partners and elucidate the molecular mechanisms of their physiological activity. We hypothesize that site specific and selectively labeled adipokines will provide novel target molecules of interaction and allow elucidating adipokine function and their roles in adipositas. In the first period of the project we will include adiponectin, vaspin, DLK1/Pref-1 and chemerin, which have been shown to play an important role in obesity and its related metabolic diseases. Proteins will be expressed recombinantly for structural analysis, and chemically modified in order to perform localization studies and identify interaction partners by co-immunoprecipitation. On the one hand, adipokines will be fluorescently labeled to follow their distribution, binding on cells and to perform in vitro assays. On the other hand, addition of biotin labels will allow co-immunoprecipitation of adipokines from cell culture supernatant or blood and modification with chelators the production of gadolinium-labeled adipokines for MRT-distribution studies in living mice. The obtained information will answer the question on the role of these important adipokines and their interplay with respect to obesity.
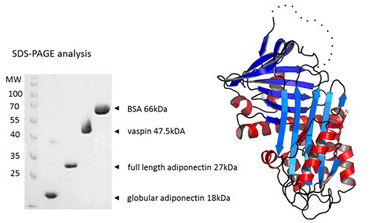
Figure 1. Expression and characterization of vaspin, full length and globular adiponectin, and crystal structure of vaspin as publishedin Heiker J et al. Cell Mol Life Sci (2013).
Fischer TF, Schoeder CT, Zellmann T, Stichel J, Meiler J, Beck-Sickinger AG. Cyclic Analogues of the Chemerin C-Terminus Mimic a Loop Conformation Essential for Activating the Chemokine-like Receptor 1. J Med Chem. 2021 Mar 25;64(6):3048-3058.
Ziffert I, Kaiser A, Hoppenz P, Mörl K, Beck-Sickinger AG. Shuttling of Peptide-Drug Conjugates by G Protein-coupled Receptors is Significantly Improved by Pulsed Application. ChemMedChem. 2020 Jul 22.
Jendrny C, Beck-Sickinger AG. Inhibition of kallikrein-related peptidases 7 and 5 by grafting serpin reactive-center loop sequences onto sunflower trypsin inhibitor-1 (SFTI-1). Chembiochem. 2016;17:719-26.
Saalbach A, Tremel J, Herbert D, Schwede K, Wandel E, Schirmer C, Anderegg U, Beck-Sickinger AG, Heiker JT, Schultz S, Magin T, Simon JC. Anti-inflammatory action of keratinocyte-derived vaspin: relevance for the pathogenesis of psoriasis. Am J Pathol. 2016;186:639-51.
Mattern A, Zellmann T, Beck-Sickinger AG. Processing, signaling and physiological function of chemerin. IUBMB Life. 2014;66:19-26.
Heiker JT, Klöting N, Kovacs P, Kuettner EB, Sträter N, Schultz S, Kern M, Stumvoll M, Blüher M, Beck-Sickinger AG. Vaspin inhibits kallikrein 7 by serpin mechanism. Cell Mol Life Sci. 2013;91:97-105.
Schultz S, Beck-Sickinger AG. Chemerin and Vaspin: Possible Targets to Treat Obesity? ChemMedChem. 2013;8:549-59.
Juhl C, Beck-Sickinger AG. Molecular tools to characterize adiponectin activity. Vitam Horm. 2012;90:31-56.
Saalbach A, Vester K, Rall K, Tremel J, Anderegg U, Beck-Sickinger AG, Blüher M, Simon JC. Vaspin-a link of obesity and psoriasis? Exp Dermatol. 2012;21:309-12.
Klöting N, Kovacs P, Kern M, Heiker JT, Fasshauer M, Schön MR, Stumvoll M, Beck-Sickinger AG, Blüher M. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia. 2011;54:1819-23.
Juhl C, Mörl K, Beck-Sickinger AG. Adiponectin receptor 1 interacts with both subunits of protein kinase CK2. Mol Cell Biochem. 2011;356:185-9.
Heiker JT, Kosel D, Beck-Sickinger AG. Molecular mechanisms of signal transduction via adiponectin and adiponectin receptors. Biol Chem. 2010;39:1005-18.
Kosel D, Heiker JT, Juhl C, Deckert CM, Blüher M, Mörl K, Beck-Sickinger AG. Adiponectin receptor 1 dimerisation is inhibited by adiponectin. J Cell Sci. 2010;123:1320-8.
Heiker JT, Klöting N, Blüher M, Beck-Sickinger AG. Access to gram scale amounts of functional globular adiponectin from E. coli inclusion bodies by alkaline-shock solubilization. Biophys Biochem Res Comm. 2010;398:32-7.
Heiker JT, Wottawah CM, Juhl C, Kosel D, Mörl K, Beck-Sickinger AG. Protein kinase CK2 interacts with adiponectin receptor 1 and participates in adiponectin signalling. Cell Signal. 2009;21:936-42.