A8 - Reversal of Hypothalamic/Midbrain Inflammation and Restoration of Central Leptin Sensitivity as a Driver of Sustained Weight Loss
The increased accessibility of palatable, calorie-dense foods represents a major predisposing environmental factor for the development of obesity. Chronic over-nutrition leads to a state of hypothalamic inflammation, central leptin and insulin resistance, subsequent hyperphagia and weight gain. Similar pathological sequelae may also take place in dopaminergic neurons in the midbrain, thereby impacting on the hedonic aspects of feeding. We have previously shown, using a rodent bariatric surgical model, that striatal dopamine signaling is modified postoperatively due to changes in intestinal nutrient sensing, and that this underlies healthier fat appetite. We will continue working with this rodent bariatric surgical model to study the possible differential influences of weight loss following surgery vs. chronic food restriction on high fat diet-induced inflammatory processes in homeostatic and hedonic feeding circuits and focus on the underlying mechanisms. We will then translate these findings by performing human studies.
Weight regain following dieting has been attributed to altered gut microbiota and adipocyte signaling. Relatively few studies have evaluated the impact of bariatric surgery on gut microbiota and adipocytes and how this may influence adipose tissue communication to the brain. We have previously shown using our rodent bariatric surgical model that thermogenic gene expression in brown adipocytes is affected, possibly underlying increased energy expenditure postoperatively. We will also take advantage of our rodent bariatric surgical model to study the possible differential influences of weight loss following surgery vs. chronic food restriction on adipocyte morphology and gene expression. Special emphasis will be placed on alterations in gut-adipose tissue signaling after bariatric surgery in order to gain mechanistic insights into how a new adiposity set-point develops. We will then translate these findings using human adipose tissue samples.
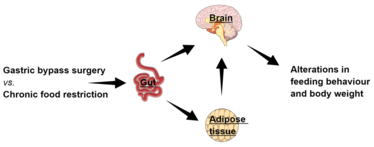
Figure 1: Gastric bypass surgery and chronic food restriction may impact differentially on gut-brain and gut-adipose tissue-brain communication through parallel and redundant pathways to exert distinct long-term influences on feeding behavior and body weight.
Chen J, Haase N, Haange SB, Sucher R, Münzker J, Jäger E, Schischke K, Seyfried F, von Bergen M, Hankir MK, Krügel U, Fenske WK. Roux-en-Y gastric bypass contributes to weight loss-independent improvement in hypothalamic inflammation and leptin sensitivity through gut-microglia-neuron-crosstalk. Mol Metab. 2021 Mar 16:101214.
Hankir MK. A sympathetic gut connection drives the metabolic benefits of Roux-en-Y gastric bypass. Cell Stress. 2020 Nov 24;4(12):265-269.
Rullmann M, Preusser S, Poppitz S, Heba S, Gousias K, Hoyer J, Schütz T, Dietrich A, Müller K, Hankir MK, Pleger B., Adiposity Related Brain Plasticity Induced by Bariatric Surgery., Front Hum Neurosci. 2019 Aug 27;13:290.
Hankir MK, Rullmann M, Seyfried F, Preusser S, Poppitz S, Heba S, Gousias K, Hoyer J, Schütz T, Dietrich A, Müller K, Pleger B. Roux-en-Y gastric bypass surgery progressively alters radiologic measures of hypothalamic inflammation in obese patients. JCI Insight. 2019 Aug 29. pii: 131329.
Seyfried F, Hankir MK. Could de-stressing the brain be the solution for long-term weight loss? Cell Stress. 2019 Jan 25;3(2):29-37.
Hankir MK, Klingenspor M. Brown adipocyte glucose metabolism: a heated subject. EMBO Rep. 2018 Aug 22. pii: e46404.
Hankir MK, Seyfried F, Miras AD, Cowley MA. Brain Feeding Circuits after Roux-en-Y Gastric Bypass.Trends Endocrinol Metab. 2018 Feb 20: S1043-2760(18)30018-3.
Hankir MK. Loading and firing the brown adipocyte. Adipocyte. 2017; Epub ahead of print.
Hankir MK, Kranz M, Gnad T, Weiner J, Wagner S, Deuther-Conrad W, Bronisch F, Steinhoff K, Luthardt J, Klöting N, Hesse S, Seibyl J, Sabri O, Heiker J, Blüher M, Pfeifer A, Brust P, Fenske WK. EMBO Mol Med. 2016;8:796-812.
Hankir MK, Bronisch F, Hintschich C, Krügel U, Seyfried F, Fenske WK. Differential effects of Roux-en-Y gastric bypass surgery on brown and beige adipose tissue thermogenesis. Metabolism. 2015;64:1240-9.
Wu Q, Li JV, Seyfried F, le Roux CW, Ashrafian H, Athanasiou T, Fenske WK, Darzi A, Nicholson JK, Holmes E, Gooderham NJ.Metabolic phenotype-microRNA data fusion analysis of the systemic consequences of Roux-en-Y gastric bypass surgery. Int J Obes (Lond). 2015; 39:1126-34.
Smith MA, Katsouri L, Irvine EE, Hankir MK, Pedroni SM, Voshol PJ, Gordon MW, Choudhury AI, Woods A, Vidal-Puig A, Carling D, Withers DJ. Deletion of S6K1 in POMC neurons impairs peripheral lipid homeostasis and leptin sensitivity in mice. Cell Rep. 2015;11:335-43.
Frost G, Sleeth ML, Arisoylu-Sahuri M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir MK, Zhang S, Carling D, Swan J, Gibson G, Viardot A, Thomas L, Bell JD. Nat Comm. 2014;5:3611.
Seyfried F, Li JV, Miras AD, Fenske WK, Cluny NL, Lannoo M, Sharkey KA, Nicholson JK, le Roux CW, Holmes E. Urinary phenotyping indicates weight loss-independent metabolic effects of Roux-en-Y gastric bypass in mice. J Proteome Res. 2013;12:1245-53.
Seyfried F, Miras AD, Fenske WK, Bueter M, Prechtl CG, Spector AC, le Roux CW. Effects of preoperative exposure to a high-fat versus a low-fat diet on ingestive behavior after gastric bypass surgery in rats. Surg Endosc. 2013;27:4192-201.
Fenske WK, Dubb S, Bueter M, Seyfried F, Patel K, Tam FW, Frankel AH, le Roux CW. Effect of bariatric surgery-induced weight loss on renal and systemic inflammation and blood pressure: a 12-month prospective study. Surg Obes Relat Dis. 2013;9:559-68.
Fenske WK, Bueter M, Miras AD, Ghatei MA, Bloom SR, le Roux CW. Exogenous peptide YY3-36 and Exendin-4 further decrease food intake, whereas octreotide increases food intake in rats after Roux-en-Y gastric bypass. Int J Obes (Lond). 2012;36:379-84.
Hankir MK, Parkinson J, Minnion M, Addison M, Bloom SR, Bell JD. PYY3-36 and Pancreatic Polypeptide differentially regulate hypothalamic neuronal activity in mice in vivo as shown by Manganese Enhanced MRI. J Neuroendocrinol. 2011;23:371-80.